Introduction:
Population studies have identified genes with germline polymorphisms associated with acute myeloid leukemia (AML) risk and outcome. However, somatic mutations in these genes have not been reported in an AML clinical population and whether they are associated with epidemiologic exposures, clinical AML phenotypes and outcome after therapy.
Methods:
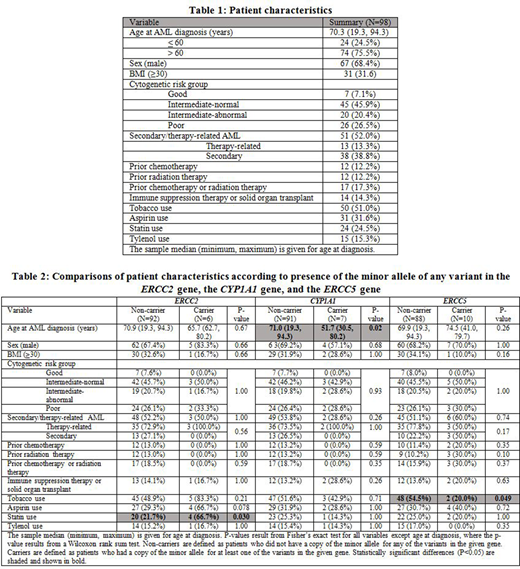
We systemically interrogated PubMed database (1998-2018), to identify genes with germline polymorphisms associated with AML risk, response to chemotherapy or outcome. To determine the prevalence and relevance of somatic mutations in these genes in an unselected AML population, we performed an analysis using Whole-Exome Sequencing (WES) on remnant diagnostic cytogenetic pellets from 98 patients from the Mayo Clinic AML Epidemiology Cohort, a detailed and highly-annotated cohort of 295 consecutive AML patients treated at Mayo Clinic Florida & Arizona between October, 2000 and December, 2011. Patient characteristics are shown in Table 1.
Samples were sequenced at a depth of ~100 million paired-end 100bp reads using Agilent SureSelectXT Human All Exon V5 + UTRs target enrichment kit. Sequencing reads were aligned to human reference genome, and somatic mutations including non-synonymous and truncating single nucleotide variants and small INDELs were identified and filtered using Exome Sequencing Project, 1000 genome, HapMap, & Mayo Clinic internal biobank genetic variants database. Copy number aberrations were identified & filtered using public copy number polymorphism databases.
The association analyses were performed at the gene level, with a primary endpoint of whether a given patient harbored a somatic mutation in any genes linked to AML risk or outcome in literature, and to determine the associations of these mutations with epidemiologic exposures, AML phenotype and clinical outcomes.
Results:
From the literature search, we identified 77 unique genes with known germline polymorphisms associated with AML risk, response to chemotherapy or outcome. Fifty-eight of these were found to be somatically mutated in our WES dataset, with subsequent analysis focusing on the 11 genes (ABCB1, CYP1A1, CYP2B6, EPHX1, ERCC1, ERCC2, ERCC5, JAK2, MEFV, MTRR, and TERT) that had greater than 5 patients with nonsynonymous somatic mutations in the given gene.
Significant associations with epidemiologic exposures and outcomes were noted in patients with somatic mutations in ERCC2, CYP1A1 and ERCC5 genes. Table 2 shows a comparison of patient characteristics and associations according to the presence of somatic mutations in these genes. Patients with mutations in CYP1A1 had a significantly younger age at AML diagnosis (Median: 51.7 vs. 71.0 years, P=.02) and significantly shorter OS in age-adjusted analysis (HR=4.45, P=.003). The former is a novel finding, whereas the latter is consistent with previous reports. Patients with mutations in ERCC2 more commonly used statins (66.7% vs. 21.7%, P=.03). Patients with ERCC5 mutations had a lower rate of tobacco use (20.0% vs. 54.5%, P=.049). In unadjusted analysis, there was a significant association between presence of somatic mutations in JAK2 and poorer survival after AML diagnosis (HR=2.83, P=.017), but this attenuated and did not retain significance when adjusting for age at AML diagnosis (HR=2.22, P=.067).
Conclusion:
Our exploratory study describes a novel association of CYP1A1 somatic nonsynonymous mutations with age of AML onset, as well as novel associations of ERCC2 and ERCC5 mutations with epidemiologic exposures in an unselect cohort of patients with AML. We confirm the association of CYP1A1 with inferior overall survival after AML diagnosis. These findings suggest that some genes associated with AML risk may also harbor somatic mutations that are clinically relevant. These results will guide a planned confirmatory prospective study to determine frequency and impact of both germline and somatic mutations of risk genes in AML patients, and may contribute to a better understanding leukemia risk assessment and potentially to prevention strategies.
Finn:Jazz Pharmaceuticals: Speakers Bureau; Celgene: Speakers Bureau; Seattle Genetics: Speakers Bureau. Cerhan:BMS/Celgene: Research Funding; NanoString: Research Funding. Foran:Revolution Medicine: Consultancy; Servier: Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Research Funding; H3Biosciences: Research Funding; Xencor: Research Funding; Trillium: Research Funding; Takeda: Research Funding; Kura Oncology: Research Funding; Aptose: Research Funding; Aprea: Research Funding; Actinium: Research Funding; Agios: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal